Making Breast Exams
Quick, Painless & AccessibleThis is Early Detection. for All.
iBreastExam is an FDA cleared hand-held device enabling primary health workers to identify breast lumps early, in just a few minutes, without any pain or radiation
2 million+ breast exams enabled in 12 countries
Health Equity, in Action
iBreastExam is enabling access to early detection like never before
Breast Cancer Screening: Can iBreastExam Bridge the Gap?
Lancet Global Health Independent Review by Nigerian Cancer Researchers
Leading Cancer Center Brings iBreastExam to Women in NY
Campaign by Montefiore Einstein Cancer Center and Rotary Club Hudson Valley
iBreastExam Presented at 2022 World Cancer Congress
"We're honored to have UE LifeSciences join UICC and the global cancer community" - Cary Adams, CEO, UICC

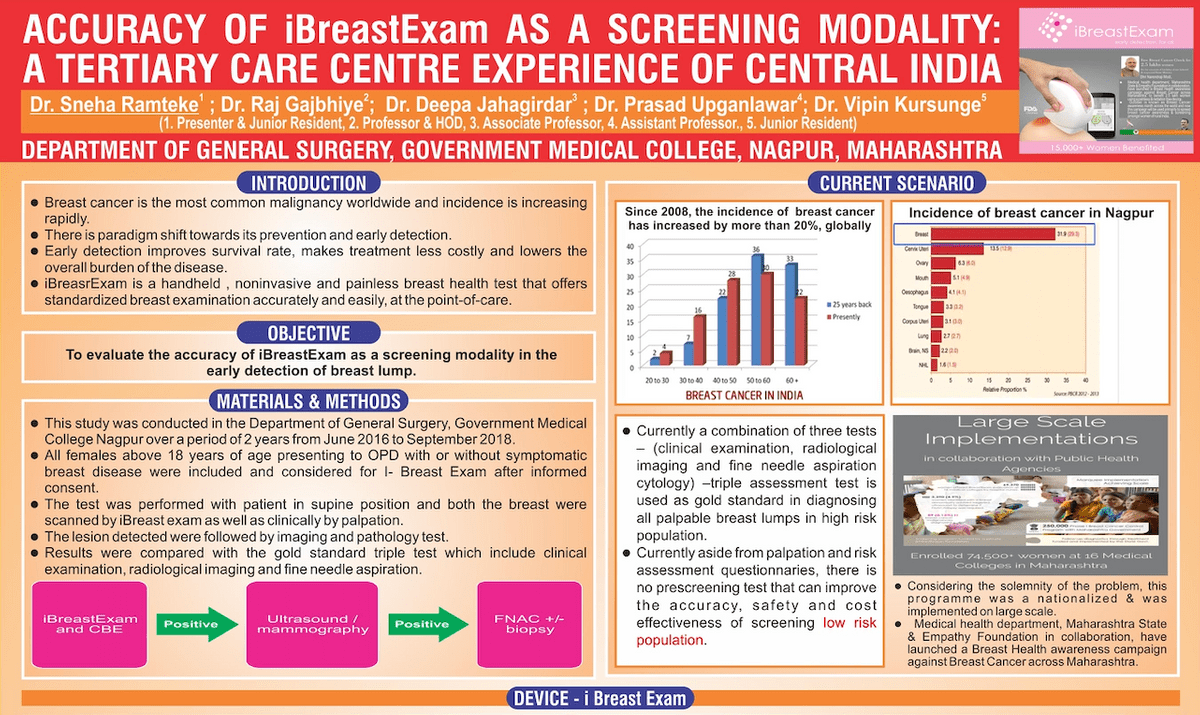
Accuracy of iBreastExam as a Screening Modality
Independent Study by Governmental Institution in 18,366 Women
US Market Launch with Siemens Healthineers
UE LifeSciences’ iBreastExam clearly addresses a well-defined niche in primary care
Large Scale Screening Campaign in Rural India
Door-to-Door campaign is bringing breast cancer early detection to 250,000 women
Real feedback
from
real women
"It's good to have first-hand information before going for a full check-up"
- Maria, Mexico
"Excellent! No Pain. Instant Results."
- Pooja, India
"It really helps to know about yourself, which we avoid in our day-to-day life"
- Jaya, Myanmar
"A step to make women feel that their health is important"
- Joomi, Botswana
Contact Us
USA - Philadelphia
India - Mumbai, Bangalore, Delhi
Malaysia - Kuala Lumpur+1.267.225.0453 (USA) +91.22.2610.2610 (India) +60.3.62063079 (Malaysia)iBreastExam is brought to you by
UE LifeSciences
Contact Us
+1 267 477 3252 (USA)
+91.22.2610.2610 (India)
+60.3.62064996 (Malaysia)
UE LifeSciences Inc. © Copyright 2023 | info@uelifesciences.com